In situ hybridisation to alpha satellite sequences¶
Contributed by Matt Lewis <hop2kin@yahoo.co.uk>
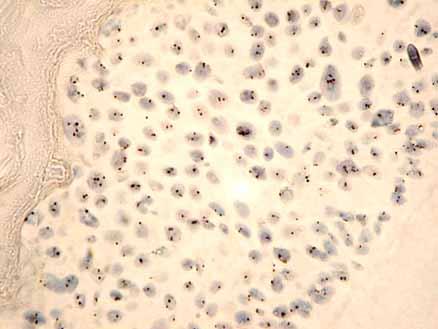
In this protocol a biotin or digoxigenin labelled DNA probe is detected using HRP-conjugated antibodies. The signal is visualised with diaminobenzidine (DAB). Normal, healthy nuclei show two spots. Aneusomic nuclei show 1, 3, 4 or more spots.
- Immerse the slides in 1M sodium thiocyanate at 80C for 10 minutes.
- Rinse in H2O and allow to dry.
- Prepare 4mg/ml pepsin as follows:
Make up 0.2M HCl [50mls H2O + 800ul conc. HCl], weigh out a little pepsin (Sigma P7012) into a universal and add the 0.2M HCl to make 4mg/ml.
- Place the slides flat in a humid chamber (eg a large square plastic petri dish) and drop the 4mg/ml pepsin gently onto the slide. Place in the 37C incubator for 30 minutes.
Pepsin digestion is the most crucial step. Too little and the probe will not be able to access the chromosomes; too much and the morphology will be disrupted.
- Rinse in H2O, 2 x 5 minutes, and allow to dry.
- Prepare the hybridisation solution.
*A multispot slide with 13mm circular coverslip needs 6ul; a square 22mm coverslip uses 10-12ul. For larger numbers place slides face to face (acting as each other’s coverslip) with 30ul solution each.
Description|Details|Quantity ———–|-------|——— Solution A|6mls de-ionised formamide, 2mls 20x SSC, 2mls H2O, 2g dextran sulphate. Store in a universal at room temperature |25ul De-ionised formamide|(Sigma F7508)|15 ul Probe|Appligene/Oncor #CP5040-B.5 (biotin labelled) or label your own probe (~10ng/ul)|5 ul Carrier DNA|Sheared salmon sperm DNA (10mg/ml)|0.5 ul H2O||2 ul 20 x SSC||2.5 ul TOTAL||50 ul*
- Apply an appropriate amount of hybridisation solution onto the slide and cover with a coverslip.
- Incubate at 80C for 10 minutes to denature the DNA
- Transfer to a humid chamber and incubate overnight at 37C
- Dip the slides into a coplin jar containing wash solution, allow the coverslips to slide off. Immerse the slides in formamide wash solution for 20 minutes at 42C.
Wash can be done without formamide at higher temperature (try 0.25 x SSC at 72C for 5 minutes)
- Immerse briefly in PBS to wash.
- Lay the slides flat in a humid chamber and drop blocking solution onto them
- Incubate for 20 minutes at room temperature.
- Drain off the blocking solution and replace with mouse anti-biotin antibody diluted 1:100 in blocking solution e.g. DAKO antibody #M0743, 100-200ul per slide.
- Incubate in a humid chamber for 40 minutes at 37C.
- Wash with blocking solution for 10 minutes
- Incubate with rabbit anti-mouse HRP (e.g. DAKO P0260 ) antibody diluted 1:80 for 40 minutes at 37C
- Wash with blocking solution for 10 minutes
- Incubate with swine anti-rabbit HRP (e.g. DAKO P0217) antibody diluted 1:100 for 40 minutes at 37C
- Wash in PBS for 5 minutes
- Make 5ml of DAB solution and then carefully drop the DAB onto the slides. DAB is carcinogenic so take care. The signal takes about 10 minutes to develop. If you’re lucky you may be able to see tiny spots at low power under the microscope (take care not to dip the lens in the DAB solution)
- Rinse in tap water, stain the nuclei using haematoxylin, de-hydrate through the ethanol series and mount in DPX.
*Duration of the haematoxylin stain depends on the haemaotoxylin and how much it has been used. Try 30 secs at first. Check under the low power microscope. *
This method is based, with permission, on an original protocol available here.