PCR product “clean-up” (SAP/EXO)¶
Contributed by Paul Barber
The purpose of this procedure is used to chew up excess primers and remove excess dNTPs from your PCR product. This proceedure is necessary to ensure clean and readable DNA sequences. Work in HIGH DNA part of lab.
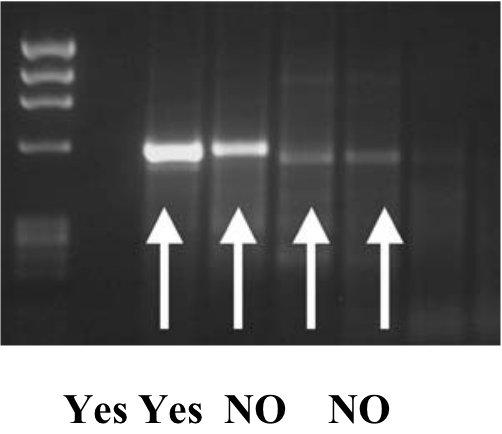
- Visualize your PCR products to make sure that your PCR worked and there are not multiple bands. A
strong amplification product (bright on the gel) will tend to sequence better. Good bands will yield good sequences, so spend the time to optimize your PCR.
- Label enough 200 L strip tubes so that there is one tube for each PCR product to be sequenced. There is
no need to sequence samples that did not amplify.
Put labels on the sides of the strip tubes, not the cap as the termocyclers heated lid will lift writing on the caps. Also, put the date and initials somewhere on the strip of tubes.
- Using a HIGH DNA pipette, add 5 L of each PCR product into a 200 L tube, changing tips for each sample.

There is nothing magic about this volume - it provides template for two sequencing reactions if the product is strong. If you need more template than this (if you have weak bands) then scale up.
- Using NO DNA pipette, make a master-mix containing the following for each PCR product to be sequenced:
- 0.5 L of shrimp alkaline phosphatase (SAP)
- 0.5 L of exonuclease I (EXO)
*So that you don’t run out, make enough mastermix for 1 extra sample per 10 samples to be digested. Therefore, if you have 9 samples, you will need 5 L of SAP and 5 L of EXO. *
- Pipette up and down to mix the SAP/EXO master mix. Ensure that it is mixed well.
- Add 1 L of master mix to each tube of template. Pipette up and down to get all SAP/EXO out of the tip.
Change tips for each sample.
- Check tubes for bubbles. If necessary, spin tubes briefly at HIGH in centrifuge.
- Set the thermocycler to SAPEXO program (if available). This program will incubate the reactions at 37 ?
C for 30 minutes, then kill the enzyme with 80 ?C for 15 minutes, and then cool the reaction to room temperature, 25 ?C. Place all samples in the machine, close the heated lid and run the program.

- The products are now ready for sequencing and can be stored at room temperature. Use 1-2 L in a 10 L sequencing reaction, depending on how bright the band was.
This method is based, with permission, on an original protocol available here.