Cresyl Violet Staining (Nissl Staining)¶
Contributed by Luke Hammond, QBI, The University of Queensland, Australia
Cresyl Violet Staining for paraffin embedded sections. Cresyl Violet Acetate solution is used to stain Nissl substance in the cytoplasm of neurons in paraformaldehyde or formalin-fixed tissue. The neuropil will be stained a granular purple-blue. This stain is commonly used to identify the neuronal structure in brain and spinal cord tissue.
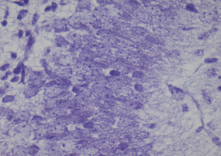
The Cresyl Violet method uses basic aniline dye to stain RNA blue, and is used to highlight important structural features of neurons. The Nissl substance (rough endoplasmic reticulum) appears dark blue due to the staining of ribosomal RNA, giving the cytoplasm a mottled appearance. Individual granules of extra-nuclear RNA are named Nissl granules (ribosomes). DNA present in the nucleus stains a similar color.
Requirements¶
95% Ethanol 70% Ethanol Differentiation solution: 2 drops glacial acetic acid in 95% ethanol Cresyl Violet Acetate 0.2% in Acetate Buffer (Fronine, Cat No: HH155)-Filtered
Method¶
- De-wax sections in xylene (2 or 3 changes of 3min each)
- Rehydrate in alcohol (100% x2), 3min each.
- Stain in 0.1% Cresyl Violet 4-15min.
- Quick rinse in tap water to remove excess stain
- Wash in 70% ethanol (the stain will be removed by this method).
- If required immerse sections for 2min in Differentiation solution – check staining on microscope.
- Dehydrate through 2x3min changes of absolute ethanol.
- Clear in xylene x2 and mount in DePeX. Allow dry in the fume hood.
This method is based, with permission, on an original protocol available here.